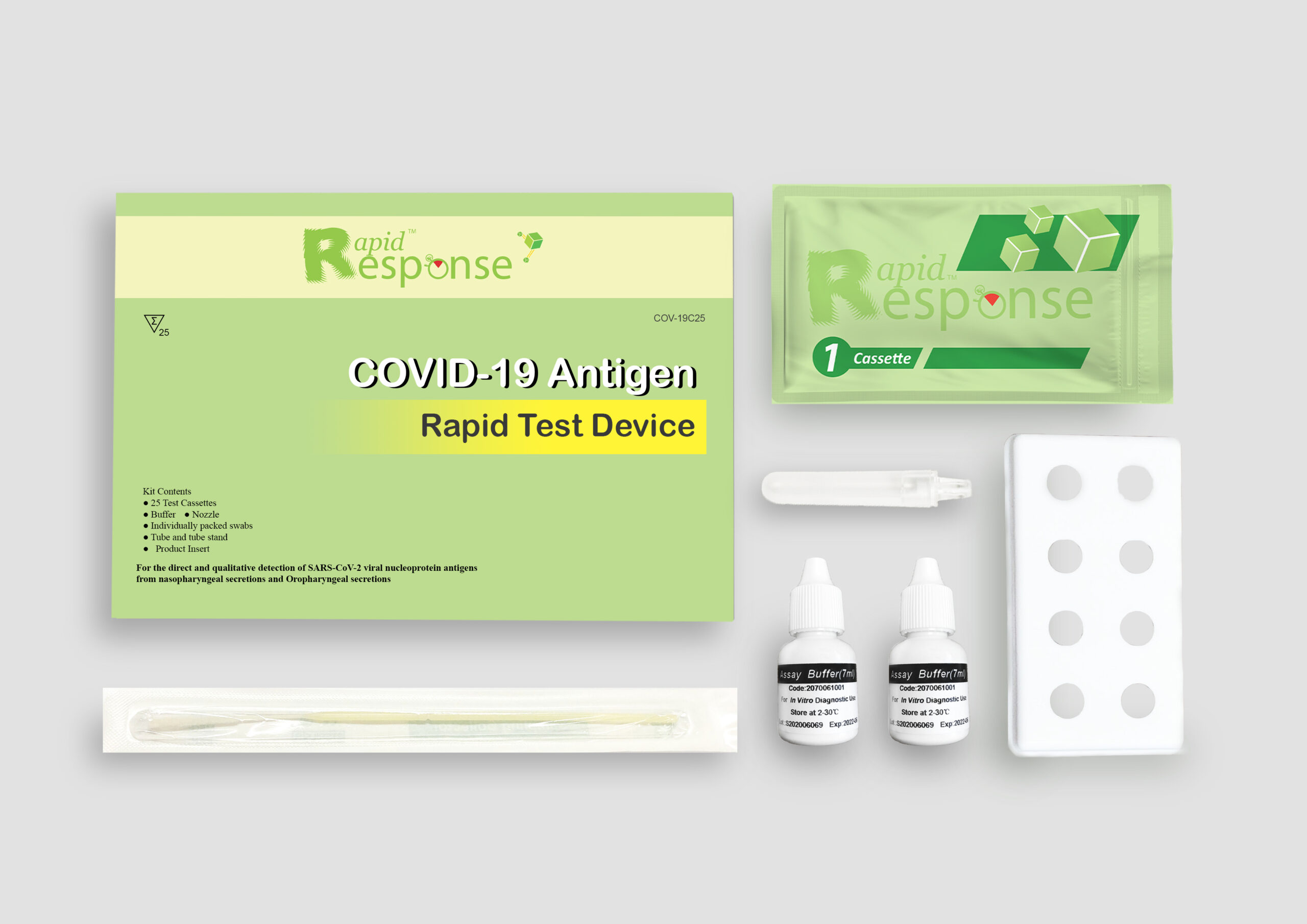
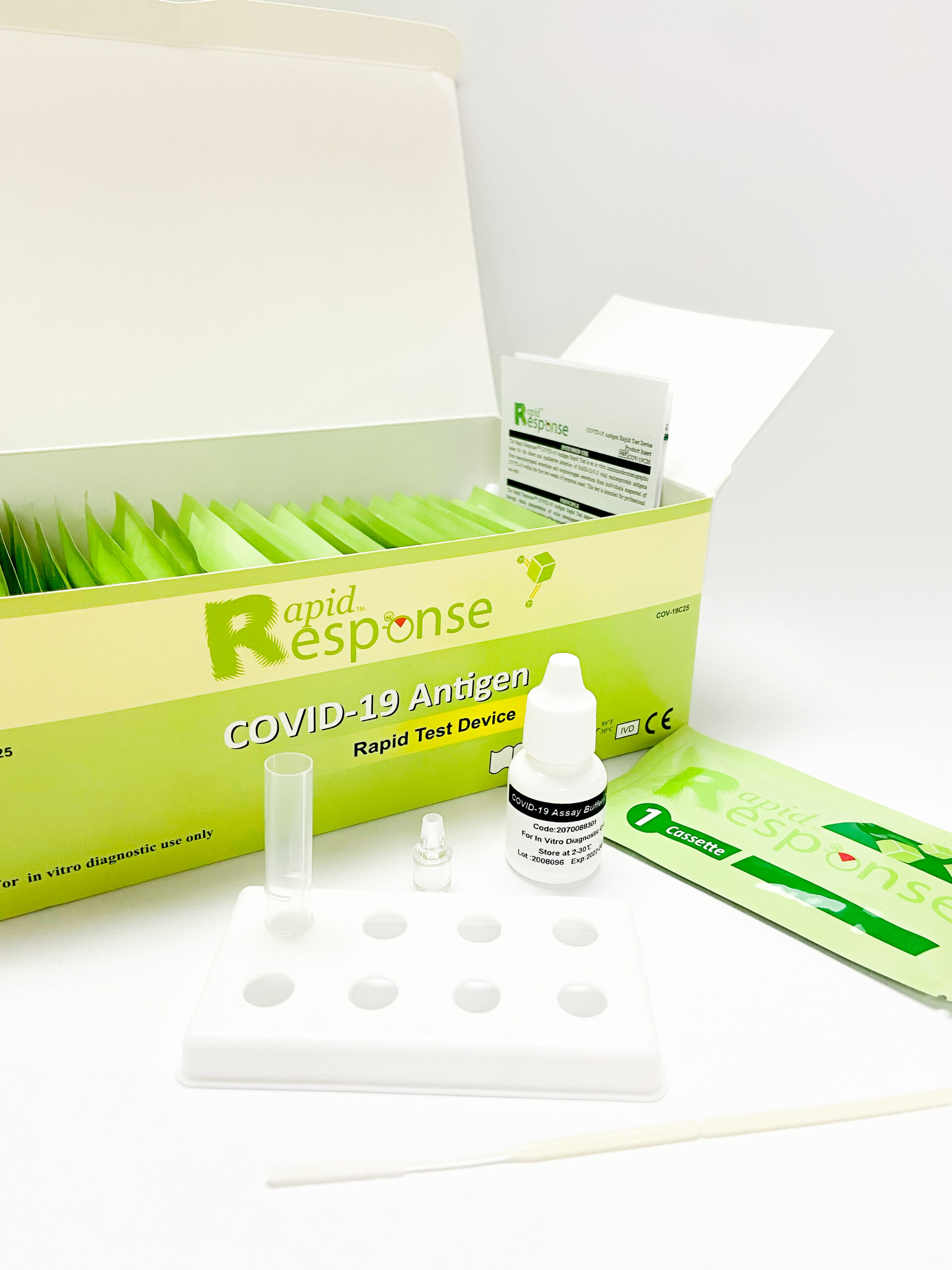
In StockRapid Response® Covid-19 Antigen Rapid Test Device
The Rapid Response® COVID-19 Antigen Rapid Test is an in vitro immunochromatographic assay for the direct and qualitative detection of SARS-CoV-2 viral nucleoprotein antigens from nasopharyngeal secretions and oropharyngeal secretions from individuals suspected of COVID-19 within the first two weeks of symptom onset.
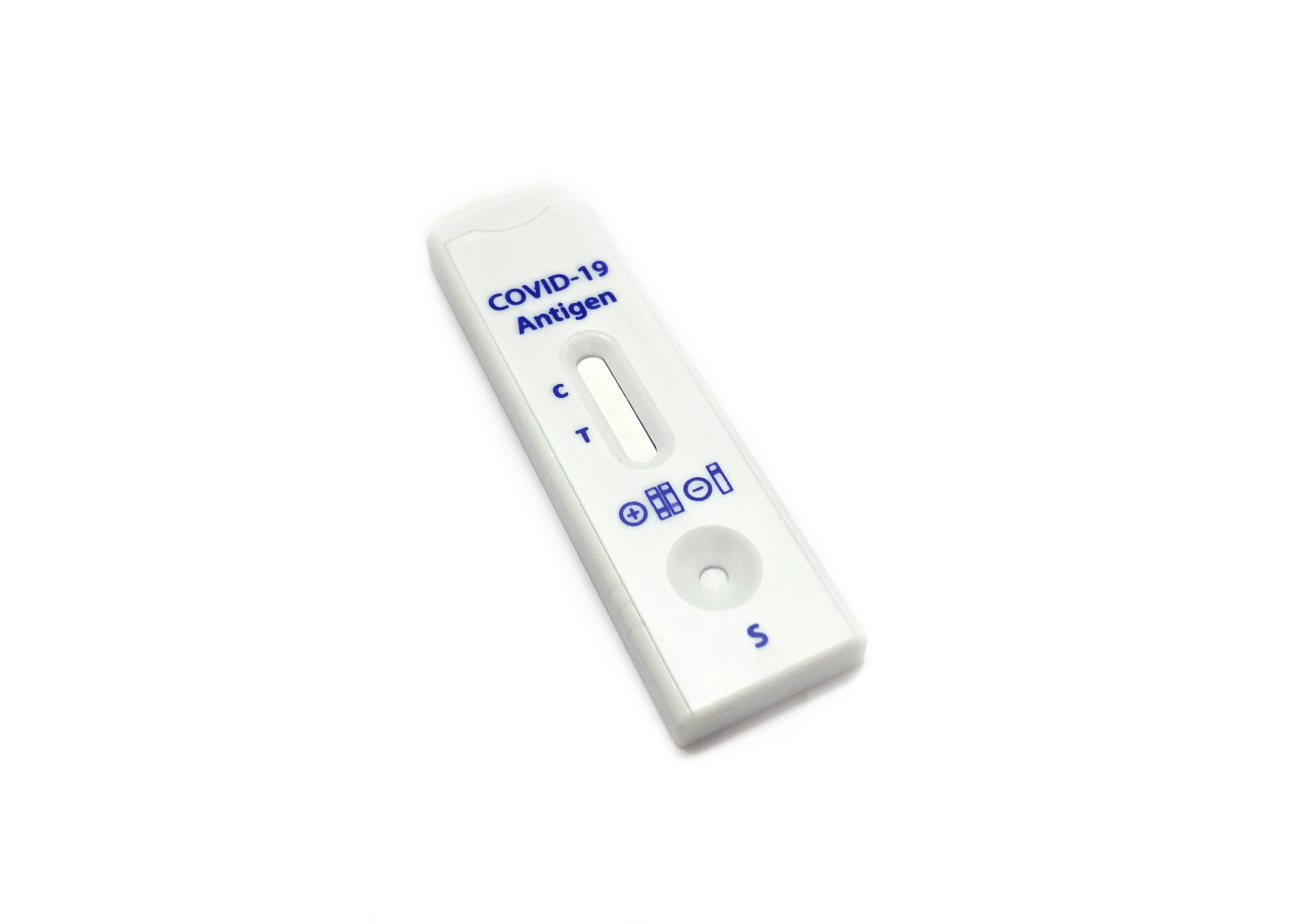
The presence of a colored band in the test region indicates a positive result for the SARS-CoV-2 viral antigens, while its absence indicates a negative result. A colored band at the control region serves as a procedural control, indicating that the proper volume of specimen has been added and membrane wicking is working.
The Kits are approved by the Pharmacy and Poisons Board of Kenya under the Ministry of Health (Kenya).
They have been fully evaluated and validated by the Kenya Medical Research Institute, with a 100% Sensitivity and Specificity (report available).
The BTNX Rapid Response Antigen Kits are approved by Health Canada, TGA in Australia, ANVISA in Brazil , and in the European Union (CE-marked).
CALL FOR PRICING AND TO PLACE YOUR ORDERS NOW….
Compare







Reviews
There are no reviews yet.